A Comprehensive Analysis of Ternary Lithium Batteries: From Principle to Application
Ternary lithium batteries, formally known as ternary polymer lithium batteries, are a type of lithium battery that utilizes lithium nickel cobalt manganese oxide (Li(NiCoMn)O₂) or lithium nickel cobalt aluminate (LiNiCoAlO₂) as the cathode material. Simply put, it is one among many lithium battery types. However, its unique cathode material formulation gives it distinct performance characteristics compared to other lithium batteries.
Against the backdrop of rapid new energy development, ternary lithium batteries play an extremely critical role, particularly in the new energy vehicle sector. As global attention to environmental protection and sustainable development continues to rise, the environmental pollution and energy crises caused by traditional fuel vehicles have become increasingly prominent, leading to the emergence of new energy vehicles.
As the “heart” of new energy vehicles, ternary lithium batteries directly determine critical metrics such as driving range, power performance, and operating costs. For instance, Tesla‘s diverse lineup leverages the high energy density of ternary lithium batteries to achieve extended range capabilities, meeting consumer demands for long-distance travel. This has propelled ternary lithium batteries to rapid prominence in the new energy vehicle market, establishing them as a mainstream choice for power batteries. Beyond new energy vehicles, ternary lithium batteries also play a vital role in energy storage. They help store surplus electricity, supporting stable grid operations and the effective utilization of renewable energy sources.
Working Principle of Lithium-ion Batteries: Basic Charging and Discharging Principles and Key Chemical Reactions
(1) Basic Charging and Discharging Principles
The working principle of lithium-ion batteries is based on the reversible movement of lithium ions between the positive and negative electrodes. During the charging process, an external power source provides energy, and lithium ions (Li⁺) are released from the positive electrode material, moving through the electrolyte; at the same time, electrons (e⁻) flow out from the positive electrode, passing through the external circuit to reach the negative electrode to maintain charge balance.
At this time, the negative electrode is in a lithium-rich state, while the positive electrode is in a lithium-poor state. To put it in a vivid way, lithium ions are like “little athletes” running back and forth between the positive and negative electrodes, “running” from the positive electrode to the negative electrode during charging. For example, when we use a charger to charge a mobile phone equipped with a ternary lithium battery, lithium ions will move within the battery according to this pattern, converting electrical energy into chemical energy for storage.
During the discharging process, the battery acts as a power source to supply electricity to the external circuit. Lithium ions are released from the negative electrode, moving through the electrolyte towards the positive electrode; electrons flow out from the negative electrode, passing through the external circuit to the positive electrode, forming a current to provide energy for the electrical equipment. In this process, chemical energy is converted into electrical energy, realizing the discharging function of the battery. Just like when an electric vehicle is driving, lithium ions in the ternary lithium battery move from the negative electrode to the positive electrode, and the released electrical energy drives the vehicle forward.
(2) Key Chemical Reactions
The anode and cathode reactions of ternary lithium batteries are the key chemical reactions for their charging and discharging functions. Taking the commonly used lithium nickel cobalt manganese oxide (Li(NiCoMn)O₂) as the cathode material and graphite (C) as the anode material as an example, the specific chemical reaction equations are as follows:
1. During Charging
Cathode reaction: Li(NiCoMn)O₂ → Li₁₋ₓ(NiCoMn)O₂ + xLi⁺ + xe⁻
Anode reaction: nC + xLi⁺ + xe⁻ → LiₓCₙ
Battery total reaction: Li(NiCoMn)O₂ + nC → Li₁₋ₓ(NiCoMn)O₂ + LiₓCₙ
In the cathode reaction, the lithium ions (Li⁺) in Li(NiCoMn)O₂ are released, along with the release of electrons (xe⁻), causing the lithium content of the cathode material to decrease and forming Li₁₋ₓ(NiCoMn)O₂. In the anode reaction, the lithium ions (xLi⁺) moving from the cathode combine with graphite (nC) to form the embedded lithium graphite compound LiₓCₙ.
2. During Discharging
Cathode reaction: Li₁₋ₓ(NiCoMn)O₂ + xLi⁺ + xe⁻ → Li(NiCoMn)O₂
Anode reaction: LiₓCₙ → nC + xLi⁺ + xe⁻
Battery total reaction: Li₁₋ₓ(NiCoMn)O₂ + LiₓCₙ → Li(NiCoMn)O₂ + nC
During discharging, the reaction direction is opposite to that during charging. In the cathode, the lithium ions (xLi⁺) and electrons (xe⁻) re-embed into Li₁₋ₓ(NiCoMn)O₂, restoring it to its original state; in the anode, the lithium ions (xLi⁺) in LiₓCₙ are deintercalated, along with the release of electrons (xe⁻).
The charge transfer in these chemical reactions is the core mechanism for the battery’s charging and discharging. During charging, the cathode undergoes an oxidation reaction, losing electrons, and the lithium ions are released from the cathode; the anode undergoes a reduction reaction, gaining electrons, and the lithium ions are embedded in the anode.
During discharging, the cathode undergoes a reduction reaction, gaining electrons, and the lithium ions are embedded in the cathode; the anode undergoes an oxidation reaction, losing electrons, and the lithium ions are released from the anode. Through such charge transfer and chemical reactions, ternary lithium batteries achieve the interconversion of electrical energy and chemical energy, providing stable power support for various equipment.
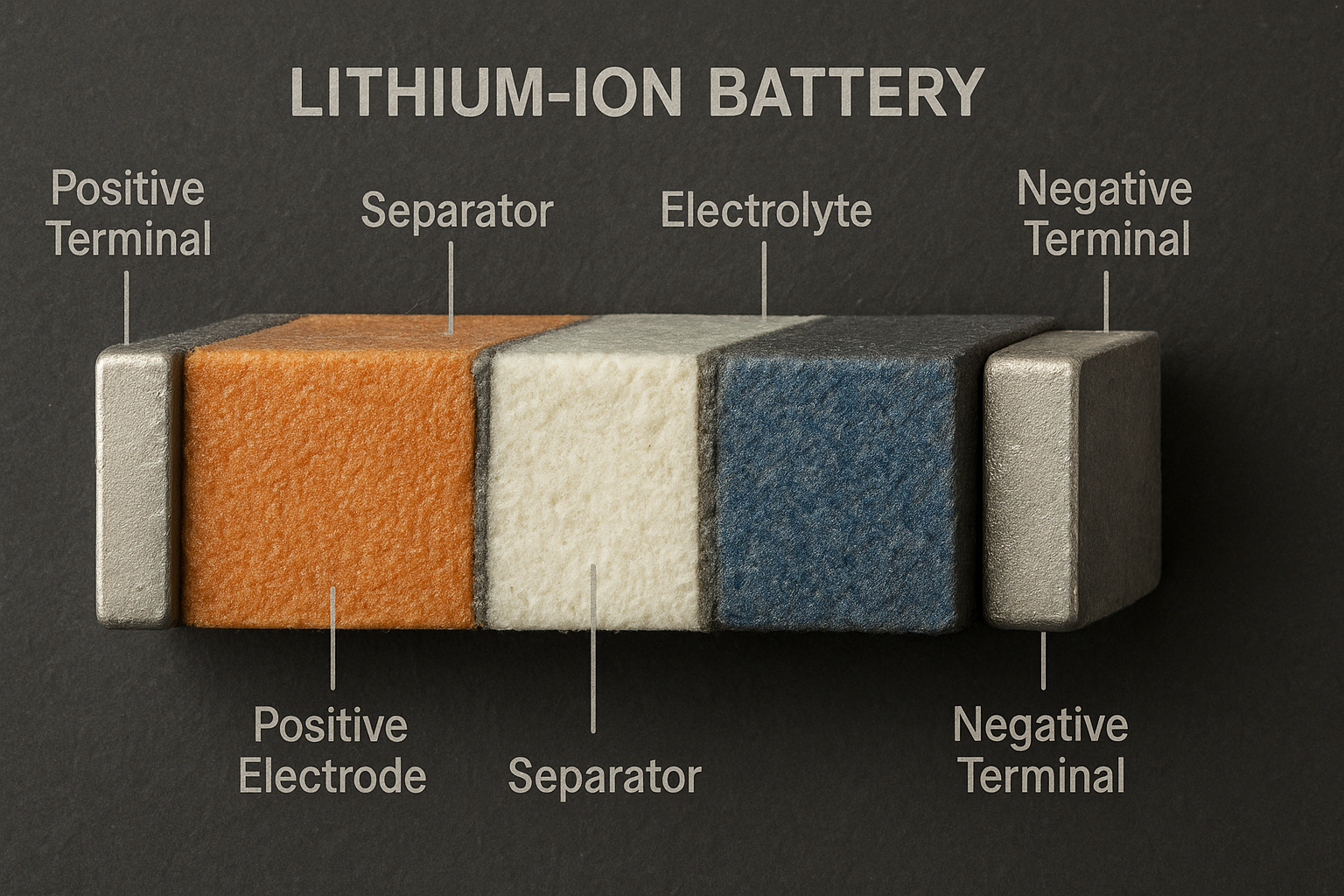
Structure Composition of Lithium-ion Batteries
The performance and safety of lithium-ion batteries are closely related to their structural composition, mainly including the positive electrode material, negative electrode material, electrolyte, and separator. Each component has clear division of labor and works in synergy.
(1) Positive Electrode Material
The positive electrode material of lithium-ion batteries is mainly composed of nickel cobalt manganese (NCM) or nickel cobalt aluminum (NCA), and the proportion of the three elements directly affects the battery performance:
Nickel (Ni): It is the key element to improve the energy density of the battery, which can increase the battery capacity, thereby extending the driving range of the device. For example, in high-nickel ternary materials, as the content of nickel increases, the volume energy density of the battery significantly improves, enabling electric vehicles to achieve longer driving ranges.
Cobalt (Co): It mainly plays a role in stabilizing the layered structure, while also improving the cycle performance and rate performance of the material, making the battery have better charge-discharge efficiency and longer service life. However, cobalt is a rare and expensive metal, and its high cost limits the large-scale application of batteries. Moreover, an excessive content of cobalt can lead to a decrease in the actual capacity of the battery.
Manganese (Mn) or Aluminum (Al): In the positive electrode material, they are mainly used to improve the safety and structural stability of the battery, as well as to reduce the material cost. For example, manganese has good electrochemical inertness, which can keep the material in a stable structure.
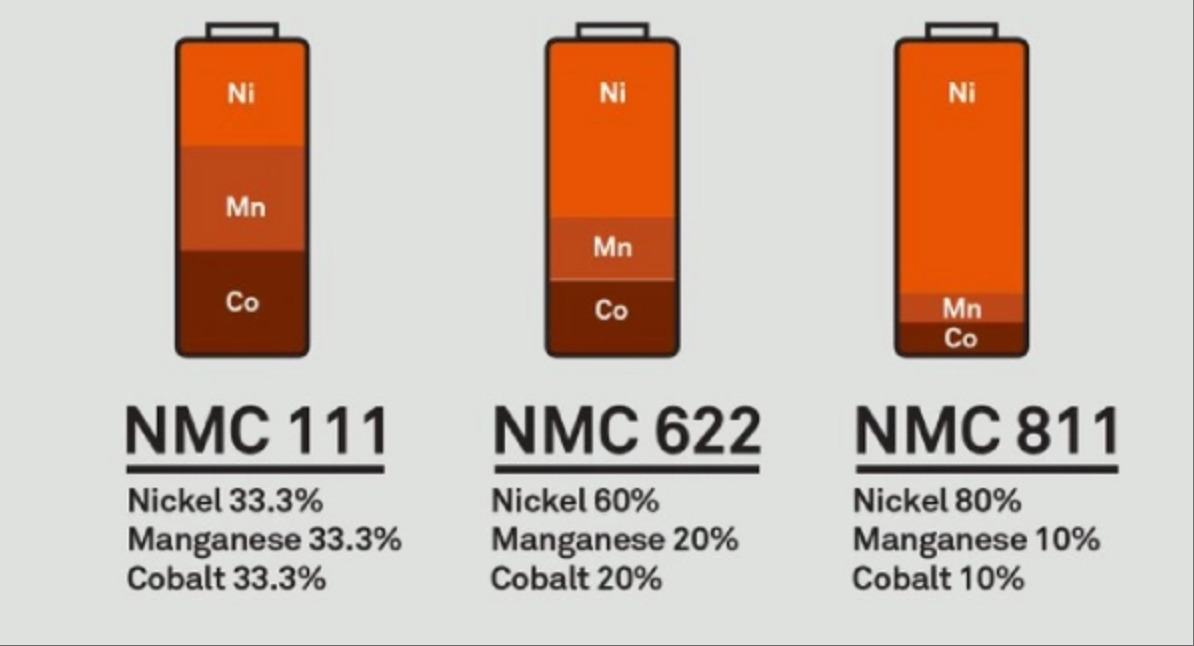
Different proportions of nickel cobalt manganese or nickel cobalt aluminum combinations will result in different battery performance. Common ternary material systems include NCM111 (nickel cobalt manganese ratio of 1:1:1), NCM523 (nickel cobalt manganese ratio of 5:2:3), NCM622 (nickel cobalt manganese ratio of 6:2:2), and NCM811 (nickel cobalt manganese ratio of 8:1:1):
The battery performance of the NCM111 system is relatively balanced, but the energy density is relatively low;
The NCM523 system achieves a good balance between energy density, cost, and safety, and was widely used in early applications;
The NCM622 and NCM811 systems have higher energy density and can meet the long-range requirements of electric vehicles. However, with the increase in nickel content, the stability and safety of the battery face certain challenges, and corresponding measures need to be taken in material preparation and battery design to improve them.
(2) Negative Electrode Materials
The negative electrode materials of ternary lithium batteries are mainly made of graphite. Graphite has good conductivity and high cycle stability, and its unique layered structure is conducive to the embedding and extraction of lithium atoms. During the charging process, after the lithium ions are desorbed from the positive electrode, they are embedded into the interlayers of the graphite negative electrode through the electrolyte, forming lithium intercalation compounds (LiₓC₆, x≤1); during discharge, the lithium ions are desorbed from the graphite negative electrode and return to the positive electrode. This embedding and desorption process can be carried out efficiently and stably, making graphite an ideal negative electrode material.
In addition, graphite also has the advantages of abundant resources and relatively low price, which makes ternary lithium batteries with graphite as the negative electrode material have certain cost competitiveness. In order to further improve the performance of the negative electrode material, researchers are also continuously exploring and developing new materials, such as silicon-based materials. The theoretical specific capacity of silicon is as high as 4200mAh/g, which is much higher than that of graphite (372mAh/g), but silicon will undergo significant volume expansion during the charging and discharging process, leading to material structure damage and degradation of cycle performance. Therefore, current research is mainly focused on how to overcome the disadvantages of silicon-based materials through material modification and composite methods, so that they can be better applied in ternary lithium batteries.
(3)Electrolyte
Electrolytes play a crucial role in the conduction of ions in ternary lithium-ion batteries, serving as an important medium for charge transfer within the battery. The electrolyte of ternary lithium-ion batteries is typically composed of lithium salts and organic solvents:
Lithium Salts: The commonly used one is lithium hexafluorophosphate (LiPF₆), which can ionize lithium ions (Li⁺) in organic solvents, providing an ion source for the battery’s charging and discharging processes;
Organic Solvents: These mainly include esters such as ethyl carbonate (EC), dimethyl carbonate (DMC), and methyl ethyl carbonate (EMC). These organic solvents have good solubility, can dissolve lithium salts, and form electrolyte solutions with high ionic conductivity.
The performance of the electrolyte directly affects the battery’s charging and discharging efficiency, cycle life, and safety, etc. High-conductivity electrolytes can enable the rapid transmission of lithium ions between the positive and negative electrodes, improving the battery’s charging and discharging rates and power performance; at the same time, the electrolyte also needs to have good chemical and thermal stability to ensure that it does not decompose or react with other battery components under various working conditions, thereby ensuring the long-term stable operation of the battery.
However, the currently commonly used electrolytes have some limitations, such as flammability and volatility, which pose certain safety risks to the battery. Therefore, the research and development of new electrolytes with higher safety performance (such as solid-state electrolytes) has become one of the research hotspots in the battery field.
(4)Separator
The separator is a key material located between the positive and negative electrodes in ternary lithium-ion batteries, mainly used to prevent direct contact between the positive and negative electrodes, which could lead to short circuits. The separator is usually made of polyolefin films or polymer composite membranes, such as polyethylene (PE), polypropylene (PP), etc. These materials have good chemical stability and mechanical strength, and can maintain stability in the complex environment inside the battery.
The separator has numerous tiny pores that allow lithium ions to pass through while blocking the passage of electrons and other substances, thus ensuring the normal operation of the battery. High-quality separators are crucial for battery performance and safety: if the separator quality is poor or there is damage, the positive and negative electrodes may come into direct contact, causing short circuits, leading to battery overheating, fires, or even explosions, which are serious safety accidents.
In practical applications, strict performance requirements are placed on separators, including thickness uniformity, porosity, pore size distribution, tensile strength, puncture strength, and thermal stability, etc. For example, higher porosity and appropriate pore size distribution can ensure the efficient transmission of lithium ions, improving the battery’s charging and discharging performance; good tensile and puncture strength can ensure that the separator does not easily break during battery assembly and use; and thermal stability requires that the separator maintain its structure and performance within the battery’s working temperature range, avoiding shrinkage, melting, or loss of isolation due to temperature rise.
Advantages of Lithium-ion Batteries
(I) High Energy Density
The energy density of lithium-ion batteries is significantly higher than that of some other common battery types. For example, compared to lithium iron phosphate batteries, the energy density of lithium iron phosphate batteries is generally around 140-160 Wh/kg, with a few advanced manufacturers reaching 180 Wh/kg; while the energy density of lithium-ion batteries is usually between 180-230 Wh/kg, and high-nickel lithium-ion batteries can even exceed 250 Wh/kg (such as CATL’s Ni55.3 high-nickel lithium-ion batteries, which stand out in terms of energy density).
Under the same volume and weight, lithium-ion batteries can store more electrical energy, which allows devices equipped with lithium-ion batteries (such as electric vehicles) to have longer driving ranges. Taking the Tesla Model 3 as an example, its long-range version equipped with lithium-ion batteries can achieve a driving range of over 600 kilometers (NEDC cycle), meeting consumers’ needs for long-distance travel and alleviating range anxiety. The higher energy density also means that in some application scenarios that have strict requirements for space and weight (such as drones, portable electronic products, etc.), lithium-ion batteries can provide more sustainable power support, improving the efficiency and user experience of the devices.
(2) Good Low-Temperature Performance
Lithium-ion batteries with ternary materials show good performance advantages in low-temperature environments. When the ambient temperature drops, the performance of many batteries is significantly affected, while lithium-ion batteries with ternary materials are relatively less affected by low temperatures. For example, in an environment of 20°C, ternary lithium-ion batteries can still maintain a certain level of charging and discharging capacity and capacity.
In the winter in the north, the temperature often drops to minus ten degrees or even lower. For electric vehicles, the low-temperature performance of the battery is particularly important. Electric vehicles equipped with ternary lithium-ion batteries can still start and operate normally in such low-temperature environments, with a relatively small decline in driving range; while some other types of batteries (such as lithium iron phosphate batteries) will see a significant drop in performance at low temperatures, leading to a significant reduction in the driving range of electric vehicles, and even failure to start normally. This gives ternary lithium-ion batteries a clear advantage in applications in cold regions, better meeting the needs of users in low-temperature environments.
(3) Excellent Cyclic Performance
Lithium-ion batteries with ternary materials have a longer cycle life. During the charging and discharging cycle, their capacity degradation is relatively slow. Generally speaking, the theoretical cycle life of ternary lithium-ion batteries can reach about 1000 to 2000 cycles. This means that after multiple charging and discharging cycles, they can still maintain good performance and provide stable power to the equipment.
Taking new energy vehicles as an example, an electric vehicle using ternary lithium-ion batteries, if it is charged and discharged once a day, based on a cycle life of 1000 cycles, theoretically it can be used for about 3 years (excluding the impact of other factors on battery life). The longer cycle life of ternary lithium-ion batteries reduces the need for frequent battery replacement during long-term use, thereby lowering the cost of use. For some energy storage systems that require long-term operation, the cyclic performance advantage of ternary lithium-ion batteries can also ensure the stable operation of the energy storage system, reducing the cost and workload of maintenance and battery replacement. At the same time, with the continuous advancement of technology, the cyclic performance of ternary lithium-ion batteries is still improving, further enhancing their value and market competitiveness.
Disadvantages of ternary lithium batteries
(1) Poor thermal stability
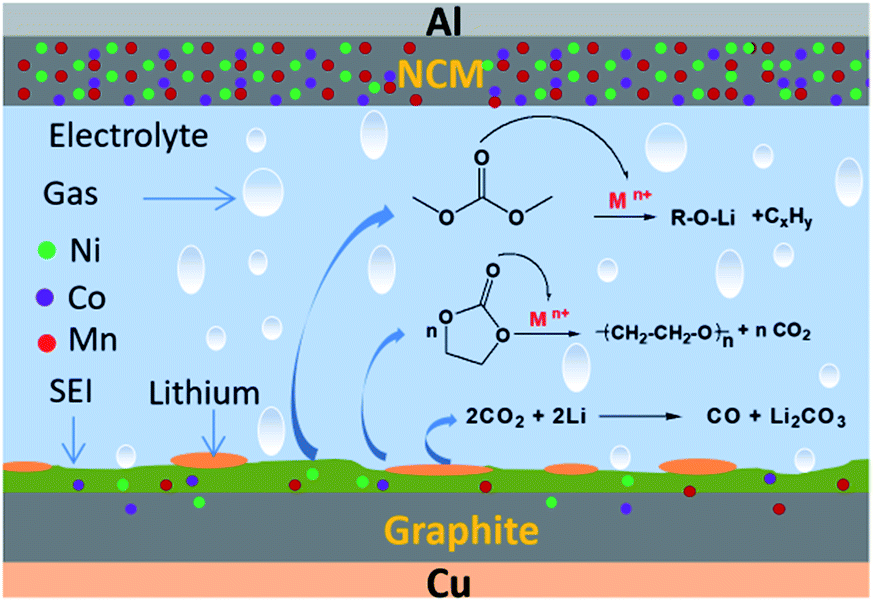
Ternary lithium batteries have poor thermal stability in high-temperature environments, which is one of their more prominent disadvantages. As the temperature rises (especially under high temperatures of 250-300℃), oxygen ions in the cathode material of ternary lithium batteries will decompose, and the oxygen molecules released will react rapidly with other substances such as electrolytes under the action of high temperature, resulting in intense oxidation-reduction reactions and generating a large amount of heat. Once this thermal runaway reaction occurs, it will rapidly intensify, causing the battery temperature to rise sharply, which can lead to serious safety accidents such as battery fires and explosions.
Under high-temperature weather conditions in summer or during long-term continuous high-current discharge of the battery, the internal temperature of the battery is prone to rise, and the problem of poor thermal stability becomes more prominent. Studies have shown that as the state of charge (SOC) of the battery increases, thermal runaway can occur at lower temperatures, and the heat released instantaneously during thermal runaway increases, making the thermal hazard greater. In practical applications, to reduce the risk of thermal runaway, it is usually necessary to equip devices with ternary lithium batteries with complex and costly thermal management systems to ensure that the battery operates within an appropriate temperature range.
(2) High Cost
The cost of ternary lithium batteries is relatively high, which largely limits their wider application. The main reason for the high cost is the presence of expensive and rare metals such as cobalt in the cathode materials. Cobalt is an important strategic resource, and the global cobalt mineral resource distribution is relatively concentrated, mainly located in a few countries such as the Democratic Republic of Congo. Factors such as unstable political situations, weak infrastructure, and technological limitations in mining in these regions lead to uncertainties in cobalt supply and significant price fluctuations.
In recent years, with the rapid development of the new energy vehicle industry, the demand for cobalt has increased significantly, further driving up cobalt prices. For example, during the period of 2021-2022, cobalt prices experienced significant fluctuations, reaching higher levels at one point, which significantly increased the raw material costs of ternary lithium batteries. In addition to cobalt, the prices of other metals such as nickel also affect the cost of ternary lithium batteries. In the cost composition of ternary lithium batteries, the cost of cathode materials accounts for a large proportion, up to 30%-40%. Due to the high cost of metals like cobalt, the cost of cathode materials remains high, thereby increasing the overall cost of the battery. Although researchers have been working hard to find ways to reduce costs (such as developing cathode materials with low cobalt or no cobalt, and optimizing production processes to reduce manufacturing costs), these methods still face many challenges in practical application, and it is difficult to achieve a significant reduction in costs in the short term.
(3) Limited Lifespan
The charging and discharging cycles of ternary lithium batteries are limited, and as the number of cycles increases, the battery capacity will gradually degrade. Generally, the theoretical cycle life of ternary lithium batteries is about 1000 to 2000 cycles. After reaching a certain number of cycles, the battery capacity may drop below 80% of the initial capacity, at which point the battery’s performance will significantly decline, and it will no longer meet the normal usage requirements of the device. For example, during the use of electric vehicles, after multiple charging and discharging cycles, the driving range of the battery will gradually shorten, affecting the user’s experience.
The reasons for battery capacity degradation mainly include the structural damage or deactivation of the positive and negative electrode materials, the decomposition of the electrolyte, and the misuse of lithium batteries: during the charging and discharging process, the positive and negative electrode materials will undergo volume changes, and after multiple cycles, it may lead to material structure damage, thereby affecting the embedding and extraction of lithium ions, reducing the battery capacity; the electrolyte will also decompose over a long period of use, producing some by-products that affect the battery’s performance; in addition, overcharging, over-discharging, high-temperature environments, and other misuse conditions will accelerate the degradation of battery capacity.
To extend the service life of lithium-ion batteries, some measures need to be taken, such as reasonably controlling the depth of charge and discharge, avoiding high-temperature environments, and using appropriate battery management systems. In daily use, try to avoid completely running out of battery or overcharging, and keep the battery operating within a suitable temperature range. These measures help to slow down the rate of battery capacity degradation and extend the service life of the battery.
Application Fields of Lithium-Ion Power Batteries
(1) Electric Vehicles
In the field of electric vehicles, lithium-ion power batteries hold a dominant position. With the global increasing attention to environmental protection and sustainable development, electric vehicles, as an important alternative to traditional fuel vehicles, have achieved rapid development. Lithium-ion power batteries, with their high energy density, good low-temperature performance, and relatively long cycle life, have become the ideal power source for electric vehicles.
Many well-known car brands have adopted lithium-ion power batteries for their electric vehicles, such as the Tesla Model 3, Model Y, and others. The lithium-ion power batteries installed in these vehicles have excellent performance in terms of driving range and power performance. Taking the long-range version of the Tesla Model 3 as an example, its driving range can reach over 600 kilometers (NEDC cycle), meeting consumers’ needs for long-distance travel and effectively alleviating range anxiety.
In addition to Tesla, traditional luxury car brands such as BMW, Mercedes-Benz, and Audi have also widely used lithium-ion power batteries in their new energy models. These brands’ electric vehicles not only continuously break through in driving range but also excel in charging speed and vehicle performance. For example, the lithium-ion power battery in the BMW i3 can quickly replenish a large amount of electricity in a short time under fast-charging mode, greatly improving user convenience.
The application of lithium-ion power batteries has promoted the rapid development of the electric vehicle industry and facilitated the transformation and upgrading of the automotive industry. It has enabled electric vehicles to gradually approach and even exceed traditional fuel vehicles in key indicators such as driving range and power performance, laying a solid foundation for the popularization and promotion of electric vehicles.
With the continuous advancement of technology, the performance of lithium-ion power batteries is still improving, and costs are gradually decreasing, which will further promote the development of the electric vehicle market and accelerate the replacement process of electric vehicles for traditional fuel vehicles.
(2) Consumer Electronics
In the field of consumer electronics, ternary lithium batteries also have extensive applications. Common devices such as smartphones, laptops, and tablets all rely on ternary lithium batteries. These devices have high requirements for battery energy density, volume, and weight, and ternary lithium batteries are exactly what meet these needs.
Taking smartphones as an example, with the continuous enrichment of phone functions and the increase in screen size, higher challenges are posed to the battery’s endurance. The high energy density of ternary lithium batteries allows smartphones to have longer endurance times under smaller volume and weight. For instance, the Apple iPhone series smartphones, after adopting ternary lithium batteries, provide users with relatively long-lasting battery support while ensuring the slim and light design of the phone, meeting the power needs of various functions such as calls, internet access, gaming, and photography in daily use.
For laptops, the application of ternary lithium batteries also significantly improves the battery life of the computers. When working or traveling, users no longer need to frequently search for power outlets, improving the convenience of work and life. For example, the Lenovo Xiaoxin Pro14 laptop, equipped with a ternary lithium battery, can support the laptop’s endurance for several hours in daily office scenarios, allowing users to work efficiently at any time. In addition, other consumer electronics such as tablets, wireless headphones, and smartwatches also benefit from the high energy density and miniaturization features of ternary lithium batteries, bringing users a better experience.
(3) Energy Storage System
In the energy storage system, ternary lithium batteries play a vital role. With the rapid development of renewable energy sources (such as solar energy, wind energy, etc.), the energy storage system has become a key to balancing energy supply and demand and improving energy utilization efficiency. Ternary lithium batteries, with their high energy density, long cycle life, and good charge-discharge performance, have been widely used in energy storage systems.
In solar power stations and wind farms, when the generated electricity exceeds the demand, the excess electricity can be stored using ternary lithium batteries; when the generated electricity is insufficient or there is no light at night or the wind is weak, the stored electricity can be released to supply power to the grid, realizing stable energy supply. For example, some large-scale solar energy storage projects have adopted large-scale ternary lithium battery energy storage systems, storing abundant solar electricity during the day and releasing it at night or during peak electricity consumption, effectively improving the utilization efficiency of solar energy and reducing dependence on traditional energy sources.
Ternary lithium batteries can also be applied in residential energy storage systems. Home users can store excess electricity generated during the day by installing solar panels and ternary lithium battery energy storage equipment, which can be used at night or during power outages, reducing home electricity costs and improving the self-sufficiency of energy. In remote areas or regions prone to power outages, ternary lithium battery energy storage systems can also serve as backup power sources, providing reliable power guarantees for local residents.
Technical Development Trends of Lithium Nickel Manganese Cobalt (NCM) Batteries
Increasing energy density is one of the important directions in the research and development of ternary lithium batteries. By optimizing the composition of cathode materials (such as further increasing the content of nickel, developing high-nickel ternary materials), the energy density of the battery can be significantly improved.
Currently, high-nickel ternary materials such as NCM811 have been widely used, and in the future, with the continuous advancement of technology, materials with higher nickel content are expected to achieve mass production. At the same time, studying new anode materials (such as silicon-based materials) is also a key to improving energy density. Although silicon-based materials have a high theoretical specific capacity, they have problems such as volume expansion. By means of material composites, nanostructure design, etc., these problems are expected to be resolved, making silicon-based materials more widely used in ternary lithium batteries.
Improving safety is also a key breakthrough in ternary lithium battery technology. In terms of thermal management technology, in-depth research into the mechanism of battery thermal runaway, development of more efficient thermal management systems, which can dissipate heat in time, prevent battery overheating and trigger safety accidents; by improving the diaphragm material and structure, enhancing the thermal stability and puncture strength of the diaphragm, it can effectively avoid positive and negative electrode short circuits and reduce safety risks; in addition, innovation in the electrode microstructure and battery appearance shape design also helps to improve the thermal safety of the battery.
Reducing costs is also an important goal of technological breakthroughs. Reducing the use of cobalt and other rare and expensive metals, developing low cobalt or cobalt-free cathode materials, is an effective way to reduce costs. For example, some companies are studying the use of other elements to replace cobalt, or developing new cobalt-free cathode materials to reduce battery costs; at the same time, optimizing production processes, improving production efficiency, and achieving mass production can also reduce the production cost per battery.
Summary
Lithium-ion batteries with ternary materials, thanks to their unique composition and working principle, have demonstrated significant advantages and broad application prospects in the new energy field. Their high energy density, good low-temperature performance, and long cycle life make them an important choice for electric vehicles, consumer electronics, and energy storage systems. However, ternary lithium-ion batteries also have disadvantages such as poor thermal stability, high cost, and limited lifespan, which to some extent limit their further development.
With the continuous advancement of technology, ternary lithium-ion batteries have made positive progress in terms of increased energy density, enhanced safety, and reduced cost. In the future, through continuous technological innovation, it is expected to overcome existing shortcomings and further improve performance and reduce costs. Driven by market demand, the application of ternary lithium-ion batteries in new energy vehicles, energy storage, and consumer electronics will be more widespread, and the market size will continue to expand.
As one of the key technologies in the new energy field, ternary lithium-ion batteries are of great significance for promoting global energy transformation and sustainable development. We look forward to the future, where ternary lithium-ion batteries can achieve greater breakthroughs in technology and the market, making greater contributions to the realization of a green, low-carbon energy future.